ViOptix laparoscopic implementation of the Intra.Ox device. Also known as “Lap.Ox”
Problem statement
Since bringing the Intra.Ox through FDA approval and into the marketplace, ViOptix has identified a user need from many of the clinics and doctors that they work with. Due to the different geometry involved in laparoscopic surgeries, clinicians were requesting that ViOptix develop a laparoscopic implementation of the Intra.Ox disposable/durable design (which would later be named the Lap.Ox). Similar to the Intra.Ox, this device features a disposable sheath enclosure and a durable (batteries are inserted into the durable this time instead of using a custom battery pack). However, various concessions in the design of the laparoscopic tube and optical polishing steps dictate that this device would have different core design and assembly process than the Intra.Ox.
My role
At first, ViOptix and Triple Ring looked into a sterilization process that uses H2O2 (run by a company called Steris) that is available inside most hospitals and would render the need for a disposable enclosure obsolete. Unfortunately, through years of testing, it was discovered that this sterilization method has an adverse and unpredictable effect on the optics of our device and could not be considered a viable long-term solution for the Lap.Ox product.
Instead, the disposable/durable concept remained crucial to the Lap.Ox design. We hypothesized internally that, in order to reach FDA 510k special approval on this device by the middle of 2025 (client expectation), Triple Ring would have to go through three iterations of the design quickly and order the last set of parts to arrive no later than June 2024.
The first design was 3D-printed and assembled in house by me and my coworker, Jordan Sweer. The assembly was fully disposable and could only be used once (i.e., durable with N=1 uses). Jordan Sweer and I learned from this first assembly and through data collection to develop the second iteration of the design, which we called pre-design verification testing, or pre-DVT.
In this phase of the project, my responsibility was part and assembly design. Due to the long lead time of injection molded plastics and printed circuit boards, the SolidWorks geometry had to be finalized, scrutinized, and ordered promptly to stay on schedule with the project. I was also responsible for doing the inventory tracking for both the durable and disposable parts, making sure we had enough parts to build 20 of each.
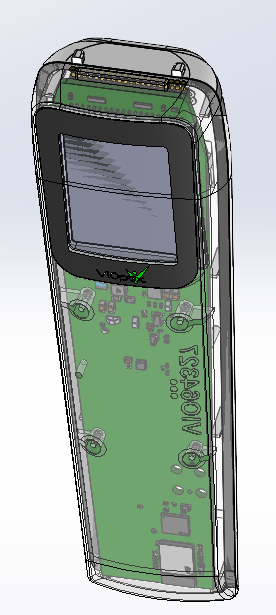
Once again, Jordan Sweer and I assembled the devices (durables and disposables separately), writing a set of durable and disposable manufacturing process instructions (MPIs) in the process. While Jordan Sweer used these first 20 units for optical testing to establish thermal cycling parameters and optical corrections during device calibration, I worked on finalizing both MPIs. Eventually, due to the volume and technical difficulty of the durable and disposable assemblies, Triple Ring would have to pass on the assembly to contract manufacturers. A CAD representation of the durable and disposable are shown below.
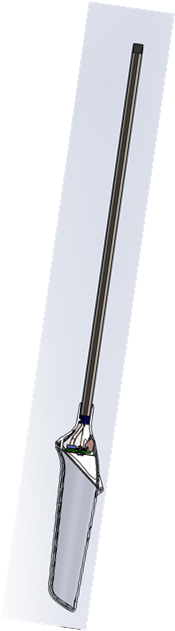
Durable (below), Disposable (right) DVT CAD representation

Disposable cross-section in CAD (right)
During the third iteration of the design, which we call the DVT units, we were responsible for building 100 durables and 300 disposables for the client. Due to the optical nature of the disposable assembly, we contracted Molex (FiberGuide LLC) to build the 300 disposables. Because the durable assembly is less technically challenging, Triple Ring enlisted our local contract manufacturer (CM), Evolve, to build these 100 units.
While I did bear the responsibility for updating the geometry for this third iteration as well as ordering enough parts to achieve our build quantities on time, my main job in this phase of the project was to act as the interface for both CMs. Because Jordan Sweer and I were the assembly experts, it was our responsibility to pass on any knowledge (including tips and tricks) that was not otherwise documented in our MPIs. It was also our responsibility to be available to debug assembly issues for either CM should they arise, and help them hunt the issue to ground. Eventually, after the DVT builds are tested and submitted to the FDA for clearance, the client intends to scale up to 1000 durables a year and 10,000 disposables a year for production. In order to get to this phase of the product, Triple Ring needs to work with both contract manufacturers to offload the BOM and purchasing aspects of building these units for clients, including the management of a complex supply chain with many long lead time parts. In the world of contract manufacturing, this is called a “turnkey” product because the CM is able to buy parts and assemble units for our client in an entirely self-sufficient manner. Because I am the author of both the durable and disposable Lap.Ox BOMs, it will be my responsibility to manage the BOM handoff for the production phase.
Project outcome
Over the course of the last 18 months, we have hit every major milestone requested by the client, and in fact, sometimes were able to exceed expectations. In the pre-DVT phase of the project, Triple Ring was able to build a nicely polished unit for ViOptix’s CEO Scott Coleridge to show off at his board meeting for live StO2 oxygen measurements.
In the current phase, due to the growing pains from switching the assembly operations from Triple Ring to Molex and Evolve, we are running slightly behind schedule procuring the 100 durables and 300 disposables. That being said, of the durable and disposable units that we have received so far, we have performed optical, mechanical, electrical, software, and destructive testing on a large portion. Thankfully, all units thus far have performed within our defined systems engineering specifications for the Lap.Ox. As of November 1, 2024, we have 150 disposables that have passed calibration and testing and are ready for clinical use. Additionally, we have 60 durables that have passed calibration and testing and are also ready for clinical use.
The DVT phase of this project has been successful in offloading the assembly cost and labor to both contract manufacturers. However, due to the time crunch of a fast approaching FDA submission for this product, Triple Ring decided to calibrate the durables and disposables in-house with an established process. The intent is to further improve and tweak both calibration processes so that by the time we reach the production phase (ViOptix is asking for an output of 10,000 disposables and 1000 durables), the calibration process can be transferred over to both contract manufacturers. This would make it possible for both CMs to deliver turnkey products that are fully calibrated and ready for use in the field, in addition to being assembled and tested for power draw.
© 2024 ariksingh.com. All rights reserved